Divergent biosynthesis of indole alkaloids FR900452 and spiro-maremycins†
Abstract
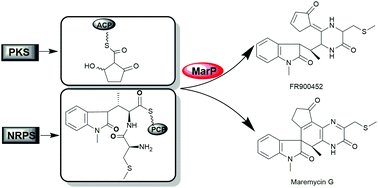
FR900452 and spirocyclic maremycins, including F and G components, are structurally related indole alkaloids, previously identified from different Streptomyces species. These alkaloids feature an indole diketopiperazine motif linked with a cyclopentenone moiety, but the linkage differs in FR900452 and the spirocyclic maremycins. Here, FR900452 and its two new analogues were identified from the fermentation broth of Streptomyces sp. B9173, the producer of maremycins. Gene inactivation and heterologous expression of the mar gene cluster confirmed that production of FR900452 shares the same biosynthetic machinery that produces maremycins. FR900452 was identified as the precursor of maremycin A/B by feeding studies. MarP, a SnoaL-like protein, was demonstrated to differentiate the biosynthesis of FR900452 from that of spiro-form maremycin G.


 Please wait while we load your content...
Please wait while we load your content...