Catalytic alkylation reactions of weakly acidic carbonyl and related compounds using alkenes as electrophiles†
Abstract
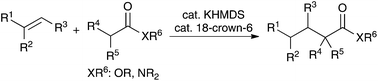
Catalytic alkylation reactions of weakly acidic carbonyl and related pronucleophiles such as amides, esters, and sulfonamides with substituted alkenes have been reported. In the presence of a strong Brønsted base catalyst system, potassium hexamethyldisilazide and 18-crown-6 ether, the desired reactions proceeded in high yields at ambient temperature with a wide substrate scope. These are atom-economical catalytic alkylation reactions of carbonyl and related compounds.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...
