Intramolecular Morita–Baylis–Hillman reaction as a strategy for the construction of tricyclic sesquiterpene cores†
Abstract
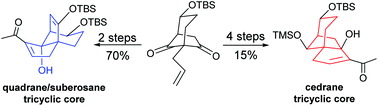
Starting from a common polyfunctionalized bicyclo[3.2.1]octane-6,8-dione intermediate, a concise synthetic route to tricyclic cores found in quadrane, suberosane, cedrane and related sesquiterpenes was developed using a Morita–Baylis–Hillman intramolecular reaction as a key step.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...