Side chain-specific 11/9-helix propensity of α/β-peptides with alternating residue types†
Abstract
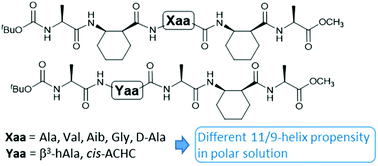
The 11/9-helix is among the most stable and non-traditional helical structures for α/β-peptides with alternating residue types. The effect of side chain groups of α-residues and β3-residues on the 11/9-helix propensity was examined under various solvent conditions. An α-amino acid residue with one of the four representative side chain groups was incorporated into the central position of an α/β-pentapeptide backbone. A β-branched valine residue did not show any destabilizing effect. α,α-Dimethylsubstituted Aib residue was tolerated under nonpolar conditions, but did not promote 11/9-helical folding. The oligomer with a glycine residue did not show 11/9-helical folding under polar solvent conditions. The single unmatched stereochemistry of D-alanine was deleterious to 11/9-helical folding. Replacement of a cyclic β-residue with an acyclic β3-residue in the 11/9-helical structure had a slight destabilizing effect, which could be compensated by a longer peptide sequence with more cyclic β-residues. These results provide a guidance for incorporating functional groups into an 11/9-helical α/β-peptide backbone to design functional oligomers.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...