Genetically-encoded fragment-based discovery (GE-FBD) of glycopeptide ligands with differential selectivity for antibodies related to mycobacterial infections†
Abstract
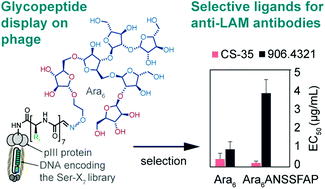
Accurate identification of tuberculosis (TB), caused by Mycobacterium tuberculosis, is important for global disease management. Point-of-care serological tests may improve TB diagnosis; however, specificities of available serodiagnostics are sub-optimal. We employed genetically encoded fragment-based discovery (GE-FBD) to select ligands for antibodies directed against the mycobacterial cell wall component lipoarabinomannan (LAM), a potent antigen. GE-FBD employed a phage displayed library of 108 heptapeptides, chemically modified with an arabinofuranosyl hexasaccharide fragment of LAM (Ara6), and the anti-LAM antibody CS-35 as a bait. The selection gave rise to glycopeptides with an enhanced affinity and selectivity for CS-35 but not for 906.4321 antibody, both of which bind to Ara6 with a comparable affinity. Multivalent assays incorporating the discovered ligands Ara6-ANSSFAP, Ara6-DAHATLR and Ara6-TTYVVNP exhibited up to 19-fold discrimination between CS-35 and 906.4321. The use of the Ara6 antigen alone failed to distinguish these antibodies. Thus, GE-FBD gives rise to ligands that differentiate monoclonal antibodies with enhanced specificity. This technology could facilitate the development of effective point-of-care serological tests for mycobacterial and other infections.


 Please wait while we load your content...
Please wait while we load your content...