Surface enhanced fluorescence and nanoscopic cell wall deformation in adhering Staphylococcus aureus upon exposure to cell wall active and non-active antibiotics†
Abstract
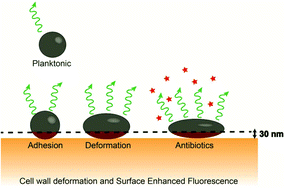
In infections, bacteria often adhere to surfaces and become deformed by the forces with which they adhere. Nanoscopic cell wall deformation defines bacterial responses to environmental conditions and is likely influenced by antibiotics. Here, staphylococcal cell wall deformation upon exposure to cell wall active and non-active antibiotics or their combinations is compared for two green-fluorescent (GFP) isogenic Staphylococcus aureus strains adhering to a gold surface, of which one lacks peptidoglycan cross-linking. Exposure to cell wall active antibiotics caused greater cell wall deformation than a buffer control in the GFP parent and in the Δpbp4GFP isogenic mutant, as measured by surface-enhanced-fluorescence. Cell wall non-active antibiotics only yielded greater deformation than a buffer control in the parent strain, while combinations of cell wall active and non-active antibiotics did not cause greater cell wall deformation. 3D-analysis of the impact of adhesion forces and Young's moduli of the cell wall, both measured using atomic force microscopy, led to the conclusion that increased deformation was mainly due to cell wall weakening and not due to the effects of antibiotics on adhesion forces. Interactions between bacteria and antibiotics are mostly studied using planktonic bacteria, while during infection, bacteria are in an adhering state that deforms their cell wall and therewith influences their adaptive responses. We anticipate that the demonstration of cell wall weakening in adhering bacteria under the influence of antibiotics and the role of peptidoglycan herein will aid in the development of new antibiotics. Surface-enhanced-fluorescence may accordingly develop into a new, highly-sensitive method for diagnosing antibiotic-resistant bacteria.


 Please wait while we load your content...
Please wait while we load your content...