Structural, functional and evolutionary perspectives on effective re-engineering of non-ribosomal peptide synthetase assembly lines
Abstract
Covering: up to May 2018
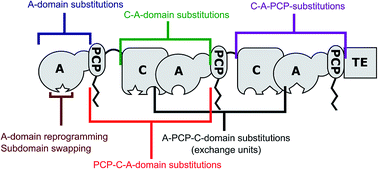
Non-ribosomal peptide synthetases (NRPSs) are mega-enzymes that form modular templates to assemble specific peptide products, independent of the ribosome. The autonomous nature of the modules in the template offers prospects for re-engineering NRPS enzymes to generate modified peptide products. Although this has clearly been a primary mechanism of natural product diversification throughout evolution, equivalent strategies have proven challenging to implement in the laboratory. In this review we examine key examples of successful and less-successful re-engineering of NRPS templates to generate novel peptides, with the aim of extracting practical guidelines to inform future efforts. We emphasise the importance of maintaining effective protein–protein interactions in recombinant NRPS templates, and identify strengths and limitations of diverse strategies for achieving different engineering outcomes.
- This article is part of the themed collection: Understanding biosynthetic protein-protein interactions


 Please wait while we load your content...
Please wait while we load your content...