Mechanistic investigation of the sulfur precursor evolution in the synthesis of highly photoluminescent Cd0.15Zn0.85S quantum dots†
Abstract
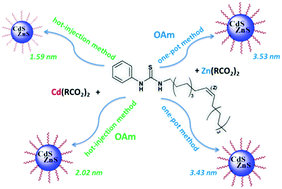
Semiconductor quantum dots (QDs) with stable and wavelength-tunable photoluminescence (PL) are very promising emitters for various photonic and optoelectronic applications. In this paper, we describe a comparison of four synthetic approaches for highly photoluminescent blue-emitting Cd0.15Zn0.85S QDs. (Z)-1-(Octadec-9-enyl)-3-phenylthiourea, initially prepared from phenyl isothiocyanate and oleylamine, was used as the source of sulfur. Environmentally friendly and commercially available N,N′-disubstituted thiourea in combination with well-known methods is a suitable precursor for the highly reproducible synthesis and further large-scale production of ternary Cd0.15Zn0.85S QDs. This study was carried out to examine the mechanism of interaction between disubstituted thiourea and metal carboxylates in one of two tautomeric forms under the influence of oleylamine, which was taken as a base in the synthesis of the QDs by hot-injection and one-pot methods. Chemical and thermal decompositions of the QDs were performed to get information about the amount and composition of organic ligands in their shells. To elucidate the mechanisms of QD formation, the organic products were studied by NMR. Optical studies showed that the prepared nanomaterials exhibit narrow emission peaks with a full width half maximum (FWHM) of around 19–33 nm and a very small size of nanocrystals (1.59–3.53 nm).


 Please wait while we load your content...
Please wait while we load your content...