New dendritic ionic liquids (DILs) for the extraction of metallic species from water†
Abstract
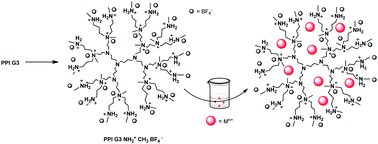
Four dendritic ionic liquids (DILs) have been easily synthesized from 3rd generation PAMAM and PPI: PAMAM G3 NH3+ Tf2N− (1), PPI G3 NH3+ Tf2N− (2), PPI G3 NH2 Me+ BF4− (3) and PAMAM G3 NH2 Me+ BF4− (4). These DILs are fully characterized by spectroscopic methods (1H, 13C and 19F NMR) and elemental analysis. Compound 1 is hydrophobic while compounds 2–4 are hydrophilic. In order to apply these DILs for the extraction of aqueous metallic cations (Cu2+, Ni2+, Pb2+, Cd2+, and Ag+), liquid–liquid extraction and dialysis were performed. ICP technology has been used to reveal the amount of metallic cation that has been extracted. Compound 1 shows the best results especially for the extraction of Cu2+, Pb2+ and Ag+ cations (up to 98%). Two processes have been employed: liquid–liquid extraction and dialysis.


 Please wait while we load your content...
Please wait while we load your content...