Effect of thermal treatment on the photocatalytic behavior of TiO2 supported on zeolites†
Abstract
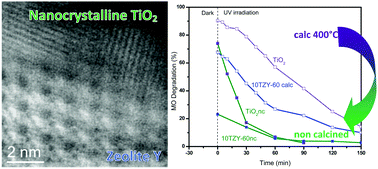
Photocatalysts based on TiO2 supported on two different synthetic zeolites (faujasite CBV 760: SiO2/Al2O3 = 60 and mordenite CBV 21A, SiO2/Al2O3 = 20), containing 10 wt% and 40 wt% TiO2 loading, have been synthesized using a sol–gel method without any further calcination. The structural, textural and optical properties of the samples have been characterized using X-ray diffraction (XRD), elemental analysis (ICP-OES), N2 adsorption–desorption isotherms, aberration corrected Scanning Transmission Electron Microscopy with High Angle Annular Dark Field imaging combined with Electron Energy Loss Spectroscopy (STEM/HAADF/EELS), and UV-Vis Diffuse Reflectance Spectroscopy (UV-Vis DRS). The characterization data confirmed that an amorphous layer containing polycrystalline anatase TiO2 was formed efficiently on the surface of the zeolites, showing 2–4 nm crystalline domains. The photocatalytic activities of different samples were tested for the photocatalytic degradation of methyl orange, which showed increased adsorption of the dye molecule, and a faster degradation reaction than the calcined counterparts. Interestingly, although the supported TiO2 catalysts were not calcined, the best catalysts, 10TZY60nc and 40TZY60nc, showed negligible Ti4+ leaching and their reusability was verified for three cycles without noticeable photocatalytic activity decrement.


 Please wait while we load your content...
Please wait while we load your content...