Structural characterization of aluminium(iii) and iron(iii) complexes of coumarinic acid in aqueous solutions from combined experimental and theoretical investigations†
Abstract
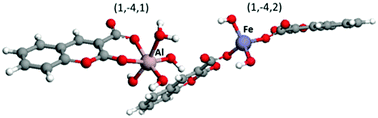
Here we have studied the complexation of coumarin-3-carboxylic acid with Al(III) and Fe(III) at 37 °C and in 0.16 mol L−1 NaCl. The protonation constant of coumarin-3-carboxylic acid was determined in order to evaluate the competition of the ligand for the metal cations and H+. Speciation profiles obtained by potentiometric titrations and supported by UV and 1H- and 13C-NMR data show that a complexation occurs at a ligand-to-Al(III) ratio of 1 : 1 and at a ligand-to-Fe(III) ratio of 1 : 2. These data were supported by a computational evaluation, which highlighted the different structural behaviour of the two metals in the complexes. Indeed, the Al(III) cation forms bidentate complexes involving a 6-membered ring by binding both the carboxyl oxygens of the ligand. In contrast, the Fe(III) ion preferentially forms monodentate tetrahedral complexes on the carboxylate moiety of the HCCA ligand.


 Please wait while we load your content...
Please wait while we load your content...