Structure-enhanced removal of Cr(vi) in aqueous solutions using MoS2 ultrathin nanosheets†
Abstract
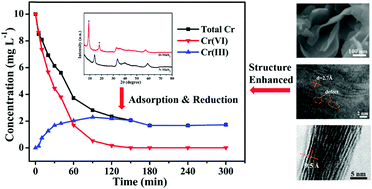
Molybdenum disulfide (MoS2) was successfully synthesized via a facile one-step hydrothermal method without addition of templates or surfactants. The obtained MoS2 product was characterized via various techniques including FESEM, HRTEM, XRD, BET and XPS analyses. Their application in capturing Cr(VI) ions in water was investigated. The results showed that the MoS2 nanosheets were ultrathin with a thickness of 5–10 nm and had a uniform lateral size of 100–200 nm. Impressively, these MoS2 nanosheets not only possessed enlarged interlayer spacing, but also had multiple defects on the basal planes, which were proved to be crucial in the removal of Cr(VI). The kinetics of chromium adsorption was found to follow the pseudo-second-order rate equation and the adsorption isotherm data were well fitted to the Langmuir model with a chromium in-take capacity of about 84.03 mg g−1. This structure-enhanced removal was likely related to the synergism of adsorption and reduction, in which the anionic Cr(VI) was adsorbed on the surface of D-MoS2via electrostatic interaction. At the same time, some fraction of the adsorbed Cr(VI) was reduced to low toxic Cr(III) and the generated Cr(III) species were adsorbed by the adsorbent. More significantly, the adsorption and reduction processes not only transferred the Cr(VI) from the aqueous environment to the surface of solid adsorbents but also alleviated the toxicity of Cr(VI). It was evident that this study may promote the application of other transition metal dichalcogenides in environmental remediation.


 Please wait while we load your content...
Please wait while we load your content...