A pyrene-benzimidazole composed effective fluoride sensor: potential mimicking of a Boolean logic gate†
Abstract
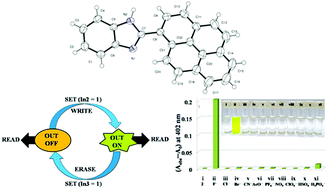
In this work, three benzimidazole derivatives incorporating 4-N,N′-dimethylaminobenzene (1), pyrene (2) and anthracene (3) moieties, respectively, have been synthesized and characterized by analytical and spectroscopic techniques. All three receptors were probed as potential anion sensors. Receptor 2 detects F− ions selectively in the presence of various anions like Cl−, Br−, CN−, ClO4−, HSO4−, NO3−, PF6−, AcO− and H2PO4− as their tetrabutylammonium salts (TBA) where the drastic color change can be observed by the naked eye while the other two molecules were found to be unresponsive towards anions. The recognition aspects have been investigated by absorption, emission and 1H NMR titration study. The sensing mechanism involves hydrogen bonding interaction between F− and an imidazole N–H proton at low concentration followed by deprotonation with the addition of excess F− ions, as confirmed by the HF2− signal in 1H NMR spectra. The strong interaction between receptor 2 and F− ion has been revealed by a high binding constant value. The limit of detection (LOD) was found to be 1.63 × 10−5 M. Based on the reversibility of the recognition event, we developed a potential “Write–Read–Erase–Read”, i.e. recyclable memory function possessing multi-write ability.


 Please wait while we load your content...
Please wait while we load your content...