A new redox-based and stepwise synthetic strategy lead to an unprecedented mixed-valence Keggin-type tungstovanadophosphate (WVI/VIV) bi-capped by vanadium(VIII)-complexes†
Abstract
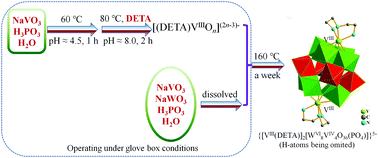
To achieve improved properties such as lower biotoxicity and better biocatalytic performances, the preparation of low oxidation state (+3) vanadium (V)-organic framework compounds (VIII-OFCs) is an important research area. Additionally, the incorporation of VIII-OFCs into a polyoxometalate (POM) is challenging due to the high standard reduction potential of VIII ions and the POM's innate sensitivity to oxidation. A new targeted and redox-based synthetic strategy has been devised to generate a V(III)-oxyanion by reducing a precursor in a pH-value controlled redox step. The V(III)-oxyanion is designed to coordinate with organic ligands for further forming an intermediate in which the metal node V(III) connects both organic units and oxygen-atoms. By employing a suitable stepwise synthesis strategy, we successfully obtained an unprecedented Keggin-type tungstovanadophosphate, (H3DETA)3(DETA){Na{[VIII(DETA)]2[WVI8VIV4O36(PO4)]}2}·2H2O (abbreviated as VIII-POWV) (DETA = diethylenetriamine, C4H13N3), bi-capped by VIII-OFCs. Its structure has been further characterized by means of powder-XRD, EDS, elemental analysis, FT-IR, XPS, thermal analysis, and SQUID magnetic measurements, etc. The seven-coordination mode (VO4N3) of VIII in VIII-POWV is uncommon and interesting. The novel structural compound, which contains mixed-valence vanadium (VIII/VIV), has better thermostability than most of the common organic POMs and shows the expected paramagnetic properties, accompanying antiferromagnetic exchange interactions under higher temperatures.


 Please wait while we load your content...
Please wait while we load your content...