Survey of short and long cuprophilic d10–d10 contacts for tetranuclear copper clusters. Understanding of bonding and ligand role from a planar superatom perspective†
Abstract
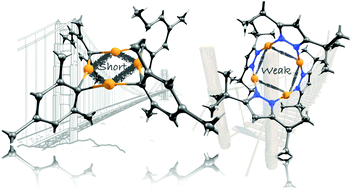
Polynuclear copper(I) complexes involving d10–d10 interactions have been studied to a lesser extent in comparison to their gold counterparts. Here, we attempt to gain a deeper understanding of ligand-protected d10 copper clusters based on density functional theory calculations, based on the evaluation of several tetranuclear copper arrays offering different cuprophilic interactions, showing short- and long-contact d10–d10 situations. Our results show that the protecting ligands display a fundamental role in the stabilization of the closed-shell central core since there is a direct relationship between ligand charge donation to the ns combinations of multinuclear metallic center and the collateral increasing of the copper–copper stabilizing interactions. Further, quantification of the incoming population of ns shell levels through a selective analysis of coefficients of its corresponding wave functions depicts a useful and novel methodology toward the characterization of the copper-metallophilic phenomena that appears in these closed-shell systems. Thereby, here we use the planar superatom approach to describe the ns valence population in terms of superatomic two-dimensional shell set levels, namely, 1s, 1px, 1py, and 1dxy. Thus, the tetranuclear clusters can be viewed as formally 8-valence electron systems, which gives a better understanding of the stable core structures. The formation of different types of cuprophilic interaction in d10–d10 Cu–Cu structures can be used to generate strongly bound closed-shell interactions in lighter counterparts from the coinage metal group, similarly to gold–gold compounds. We expect that this analysis can be extended to linear and polymeric d10–d10 Cu–Cu arrays in order to gain a deeper understanding of the closed-shell bonding situation.


 Please wait while we load your content...
Please wait while we load your content...