Transformation of the surface compositions of titanium during alkali and heat treatment at different vacuum degrees
Abstract
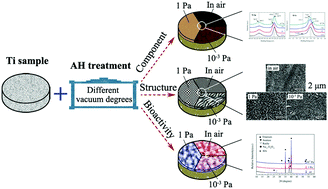
In this study, the alkali and heat treatment (AH) of titanium (Ti) at different ambient pressures (at atmospheric pressure, 1 Pa and 10−3 Pa) was studied. The results demonstrated that under atmospheric conditions porous layers with rutile/anatase TiO2 formed on the Ti surface, and at 1 Pa and 10−3 Pa more loose surface structures with a large pore size and sodium titanate (Na1.7Ti6O11) were obtained. In particular, after AH at 1 Pa, the surface layer exhibited greater roughness, better wettability and easier hydroxyapatite (HA) deposition in simulated body fluid (SBF) than under other conditions. The electrochemical characterization revealed the porous network structure on the Ti surfaces formed during AH, which resulted in a successive decrease in the corrosion resistance in the SBF with increasing vacuum degrees. This investigation of the AH technique of Ti at different chamber pressures would promote a more potential clinical application of the AH method.


 Please wait while we load your content...
Please wait while we load your content...