An efficient electrode for simultaneous determination of guanine and adenine using nano-sized lead telluride with graphene†
Abstract
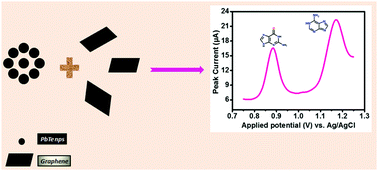
Herein, lead telluride (PbTe) nanocrystals were chemically synthesized at room temperature via reduction of homogeneous mixtures of tartrate complexes of Pb2+ and Te4+ with sodium borohydride. Graphene (GR) was synthesized through thermal reduction of graphene oxide (GO) in the presence of Zn dust. The structure, phase, and morphology of the synthesized PbTe and GR were characterized by XRD, FESEM, and EDX. Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) were used to study the electro-oxidation behaviors of guanine and adenine molecules on the surface of the composite of lead telluride and graphene-modified graphite paste electrode (PbTe/GRGP). The voltammetric responses revealed that the electrocatalytic properties of these biomolecules (GU and AD) were significantly improved after incorporation of both PbTe nanoparticles and graphene into graphite powder in comparison with those of bare graphite (bare GP) and the graphene graphite paste electrode (GR/GP). CV studies indicate well defined and distinct irreversible oxidation peaks for both biomolecules upon treatment of the PbTe/GRGP electrode with a solution of biomolecules in phosphate buffer (pH 5). DPV measurements illustrated the appearance of two well defined oxidation peaks of GU and AD with a peak potential separation of 289 mV. Under optimized experimental conditions, the modified electrode exhibited wider linear ranges of 4–140 μM and 3–50 μM with the detection limits of 80 nM (S/N = 3) and 70 nM for GU and AD, respectively. Furthermore, the modified electrode was successfully used in spiked human urine and serum samples to determine the GU and AD contents for real time applications.


 Please wait while we load your content...
Please wait while we load your content...