A β-carboline derivative-based nickel(ii) complex as a potential antitumor agent: synthesis, characterization, and cytotoxicity†‡
Abstract
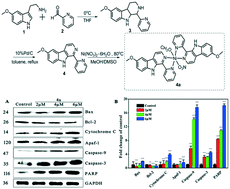
A novel nickel(II) complex of 6-methoxy-1-pyridine-β-carboline (4a) was synthesized and characterized. The cytotoxicities of the complex towards six cancer cell lines, including MGC-803, Hep G2, T24, OS-RC-2, NCI-H460, and SK-OV-3, and human normal liver cell line HL-7702 were investigated. The IC50 values for MGC-803, Hep G2, T24, OS-RC-2, NCI-H460 and SK-OV-3 were generally in the micromolar range (3.77–15.10 μM), lower than those of ligand 4 and cisplatin. Furthermore, 4a (6 μM) significantly induced cell cycle arrest at the S phase, and caused the down-regulation of p-AKT, cyclin E, cyclin A and CDK2 and the up-regulation of p27. Various experiments showed that 4a induced apoptosis, activated caspase-3, increased the levels of reactive oxygen species (ROS) and enhanced the intracellular [Ca2+]c levels in MGC-803. In addition, the expression of intrinsic apoptotic proteins, including cytochrome c and apaf-1, increased. Further intrinsic apoptosis was triggered via executive molecular caspase-9 and caspase-3. In short, 4a exerted its cytotoxic activity primarily through inducing cell cycle arrest at the S phase and intrinsic apoptosis.


 Please wait while we load your content...
Please wait while we load your content...