A fluorescent microbead-based microfluidic immunoassay chip for immune cell cytokine secretion quantification†
Abstract
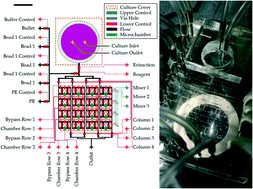
Quantitative and dynamic analyses of immune cell secretory cytokines are essential for precise determination and characterization of the “immune phenotype” of patients for clinical diagnosis and treatment of immune-related diseases. Although multiple methods including the enzyme-linked immunosorbent assay (ELISA) have been applied for cytokine detection, such measurements remain very challenging in real-time, high-throughput, and high-sensitivity immune cell analysis. In this paper, we report a highly integrated microfluidic device that allows for on-chip isolation, culture, and stimulation, as well as sensitive and dynamic cytokine profiling of immune cells. Such a microfluidic sensing chip is integrated with cytometric fluorescent microbeads for real-time and multiplexed monitoring of immune cell cytokine secretion dynamics, consuming a relatively small extracted sample volume (160 nl) without interrupting the immune cell culture. Furthermore, it is integrated with a Taylor dispersion-based mixing unit in each detection chamber that shortens the immunoassay period down to less than 30 minutes. We demonstrate the profiling of multiple pro-inflammatory cytokine secretions (e.g. interleukin-6, interleukin-8, and tumor necrosis factors) of human peripheral blood mononuclear cells (PBMCs) with a sensitivity of 20 pg ml−1 and a sample volume of 160 nl per detection. Further applications of this automated, rapid, and high-throughput microfluidic immunophenotyping platform can help unleash the mechanisms of systemic immune responses, and enable efficient assessments of the pathologic immune status for clinical diagnosis and immune therapy.


 Please wait while we load your content...
Please wait while we load your content...