Imidazolium-based dicationic ionic liquids: highly efficient extractants for separating aromatics from aliphatics†
Abstract
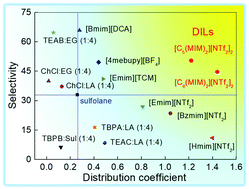
The separation of aromatics from aliphatics is challenging and energy-consuming because of the very close boiling points among these compounds and the possibility of forming azeotropes. In this work, we designed two imidazolium-based dicationic ionic liquids, [C5(MIM)2][NTf2]2 (DIL1) and [C6(MIM)2][NTf2]2 (DIL2), and found that they could highly efficiently extract aromatics from aliphatics. Three different aromatic and aliphatic mixtures, toluene/n-heptane, benzene/n-hexane and benzene/cyclohexane, were used to investigate the universality of DILs. The extractive performance of both DILs was compared with those of other promising ILs and sulfolane. The mixtures of toluene and aliphatics with different chain lengths were also investigated. The results showed that the two DILs had relatively high selectivity and distribution coefficients for different aromatic and aliphatic mixtures. At 30 °C, the highest values of selectivity and distribution coefficient for toluene/n-heptane, benzene/n-hexane and benzene/cyclohexane systems were 50.4, 64.8, and 24.9 and 1.44, 2.44, and 2.25, respectively. The performance of DILs at 30 °C was better than that at 40 °C. Besides, a higher selectivity could be obtained when toluene is used with a longer chain aliphatic, such as a selectivity of 61.4 could be obtained when the aliphatic was n-nonane. The study of the physicochemical properties of DILs showed that they were more stable than the previous ones. In a word, the two DILs were evaluated as potential solvents for the separation of aromatics from aliphatics.


 Please wait while we load your content...
Please wait while we load your content...