Ionic liquids tailored and confined by one-step assembly with mesoporous silica for boosting the catalytic conversion of CO2 into cyclic carbonates†
Abstract
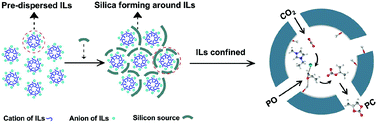
On the basis of economic and energy-saving criteria, the minimum effective dose of ionic liquids (ILs) for the catalytic conversion of CO2 into cyclic carbonates was first explored by confinement. With one-step assembly of mesoporous silica (mSiO2) by using a fixed amount of silicon source with varying amounts of ILs as templates, certain amounts of 1-ethyl-3-methyl imidazolium bromides (EmimBr) were tailored and confined in mSiO2. The confined ILs (EmimBr@mSiO2) retained the advantages of homo- and heterogeneous catalysts, exhibiting higher performance than bulk EmimBr under identical reaction conditions. Amongst all the prepared materials, EmimBr@mSiO2 with the lowest amount of EmimBr (6.9 wt%) exhibited the biggest improvement in catalytic activity, achieving a TOF of 112.6 h−1 which is almost 1.7 times the bulk phase with good recyclability. The boosted catalytic activity could be attributed to the larger proportion of mesopores, the better dispersity of EmimBr (Si/Br = 25) and the synergistic effect from more exposed silanol groups (Si–OH/Br = 8) in the structure. The good recyclability was then explained by the XPS analysis and density functional theory (DFT) calculation, which confirmed that the compressing effect from Si–OH could enhance the cation and anion interaction to stabilize ILs in the space more firmly when less ILs were confined.


 Please wait while we load your content...
Please wait while we load your content...