A CaMnAl-hydrotalcite solid basic catalyst toward the aldol condensation reaction with a comparable level to liquid alkali catalysts†
Abstract
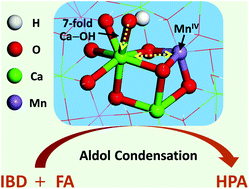
In a number of heterogeneous catalysis processes, the promotion effect toward active sites is of vital importance and remains a challenge to obtain largely-improved catalytic performance. Herein, rehydrated Ca4MnxAl-layered double hydroxides (denoted as re-Ca4MnxAl-LDH) were prepared based on a structure memory effect of LDH precursors, which exhibited extremely high heterogeneous catalytic performance for the aldol condensation reaction, with the assistance of the promotion effect of Mn. A combination study including XRD, EXAFS, XPS, CDCl3-FTIR and DFT calculations confirms that re-Ca4MnxAl-LDH samples with Ca–O–MnIV structure show a highly-exposed Ca2+ s-orbital and strengthened coordination between Ca2+ with 7-fold OH−, providing a weakened Brønsted basic site compared with the reference sample re-Ca4Al-LDH. The optimized re-Ca4Mn0.5Al-LDH catalyst exhibits prominent catalytic performance toward the condensation of isobutyraldehyde (IBD) and formaldehyde (FA) to produce hydroxypivaldehyde (HPA), with a HPA yield of 70.3%. This is significantly higher than re-Ca4Al-LDHs (63.3%) and even comparable to the level of liquid alkali catalysts (73.2%). Studies on the structure–property correlation reveal that the weakened basic site originating from Ca–O–MnIV serves as a promoted active center, which accelerates the product desorption and thus largely improves HPA selectivity. This promoted re-Ca4Mn0.5Al-LDH catalyst can be potentially applied as a promising candidate in heterogeneous aldol condensation reactions.


 Please wait while we load your content...
Please wait while we load your content...