Simultaneous elimination of cationic uranium(vi) and anionic rhenium(vii) by graphene oxide–poly(ethyleneimine) macrostructures: a batch, XPS, EXAFS, and DFT combined study†
Abstract
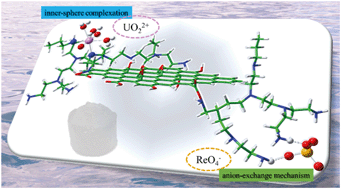
In the field of radioactive wastewater treatment associated with environmental remediation, a big challenge is to achieve the simultaneous elimination of toxic metal cations and metallate anions. Herein, a three-dimensional (3D) graphene oxide-supported ethyleneimine polymer composite (GO–PEI) was synthesized by a self-assembly strategy and used for the simultaneous removal of cationic U(VI) and anionic Re(VII), which acts as a surrogate for Tc(VII), from aqueous solution. The maximum adsorption capacity of GO–PEI composites at pH 5.0 for U(VI) and at pH 3.5 for Re(VII) was determined to be 629.5 and 262.6 mg g−1, respectively. Based on Fourier transform infrared (FT-IR) spectroscopy, X-ray photoelectron spectroscopy (XPS), extended X-ray absorption fine structure (EXAFS) analyses, and density functional theory (DFT) calculations, the adsorption of U(VI) is predominantly attributed to the coordination with abundant amino and oxygen-containing groups anchored on the hydrogel. In contrast, the removal of Re(VII) is correlated with the anion-exchange mechanism. In addition, GO–PEI has demonstrated a highly extractive adsorption capability toward U(VI) in ultralow concentrations. Our work may pave the way for creating an exciting new category of material with versatile capabilities for the treatment of radioactive effluent from the nuclear fuel cycle.


 Please wait while we load your content...
Please wait while we load your content...