Layered LiTiO2 for the protection of Li2S cathodes against dissolution: mechanisms of the remarkable performance boost†
Abstract
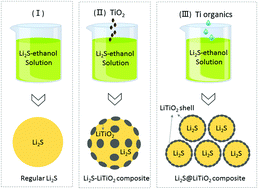
Lithium sulfide (Li2S) cathodes have been viewed as very promising candidates for next-generation lightweight Li and Li-ion batteries. Prior work on the deposition of carbon shells around Li2S particles showed reduced dissolution of polysulfides and improved cathode stability. However, due to the substantial volume changes during cycling and the low chemical binding energy between carbon and sulfides, defects almost inevitably forming in the carbon shell during battery operation commonly lead to premature cell failure. In this study, we show that conformal coatings of layered LiTiO2 may offer better protection against polysulfide dissolution and the shuttle effects. Density functional theory (DFT) calculations revealed that LiTiO2 exhibits a strong affinity for sulfur species (Li2Sx) and, most importantly, induces a rapid conversion of longer (highly soluble) polysulfides to short polysulfides, which exhibit minimum solubility in electrolytes. Quite remarkably, even the mere presence of the electronically conductive layered oxides (LiMO2, M = metal) such as LiTiO2 in the cathodes (e.g., as a component of the mix with Li2S) enhanced the cell rate and cycling stability dramatically. Advanced material characterization in combination with quantum chemistry calculations provided unique insights into the mechanisms of the incredible performance boost, such as interactions between Li2Sx and the LiTiO2 surface, leading to breakage of S–S bonds.


 Please wait while we load your content...
Please wait while we load your content...