Effect of electrolyte on the nanostructure of the solid electrolyte interphase (SEI) and performance of lithium metal anodes†
Abstract
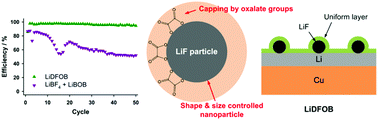
Developing electrolytes that enable commercially viable lithium metal anodes for rechargeable lithium batteries remains challenging, despite recent exhaustive efforts. Electrolytes of similar composition, yet different structure, have been investigated to understand key mechanisms for improving the cycling performance of lithium metal anodes. Specifically, the electrolytes investigated include LiPF6, LiBF4, lithium bis(oxalato)borate (LiBOB), and lithium difluoro(oxalato)borate (LiDFOB) dissolved in a mixture of ethylene carbonate (EC) and ethyl methyl carbonate (EMC). There is a remarkable difference in the cycling performance of 1.2 M LiDFOB in EC : EMC (3 : 7) compared to 0.6 M LiBF4 + 0.6 M LiBOB in EC : EMC (3 : 7), despite the effectively equivalent chemical composition. The LiDFOB electrolyte has significantly better cycling performance. Furthermore, the chemical compositions of the SEI generated on the lithium metal electrode from the two electrolytes are very similar, especially after the 1st plating, suggesting that the chemical composition of the SEI may not be the primary source for the difference in cycling performance. Ex situ transmission electron microscopy (TEM) reveals that the difference in cycling performance can be traced to the presence of nanostructured LiF particles in the SEI from the LiDFOB electrolyte. It is proposed that the capping ability of the oxalate moiety from LiDFOB, in combination with simultaneous generation of LiF, leads to generation of uniform and evenly distributed nanostructured LiF particles. The presence of nanostructured LiF in the SEI results in uniform diffusion field gradients on the lithium electrode which leads to improved cycling performance. The proposed mechanism not only provides insight for improving lithium metal anodes for batteries, but also expands upon the understanding of the role of LiF in the SEI on graphite electrodes in commercial lithium ion batteries. A superior understanding of the structure and function of the SEI will facilitate the development of next-generation energy storage systems.


 Please wait while we load your content...
Please wait while we load your content...
