Iodine chemistry determines the defect tolerance of lead-halide perovskites†
Abstract
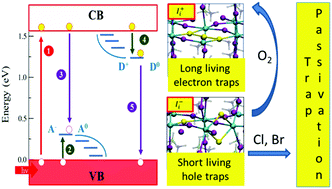
Metal-halide perovskites are outstanding materials for photovoltaics. Their long carrier lifetimes and diffusion lengths favor efficient charge collection, leading to efficiencies competing with established photovoltaics. These observations suggest an apparently low density of traps in the prototype methylammonium lead iodide (MAPbI3) contrary to the expected high defect density of a low-temperature, solution-processed material. Combining first-principles calculations and spectroscopic measurements we identify less abundant iodine defects as the source of photochemically active deep electron and hole traps in MAPbI3. The peculiar iodine redox chemistry leads, however, to kinetic deactivation of filled electron traps, leaving only short-living hole traps as potentially harmful defects. Under mild oxidizing conditions the amphoteric hole traps can be converted into kinetically inactive electron traps, providing a rationale for the defect tolerance of metal-halide perovskites. Bromine and chlorine doping of MAPbI3 also inactivate hole traps, possibly explaining the superior optoelectronic properties of mixed-halide perovskites.


 Please wait while we load your content...
Please wait while we load your content...