Solar desalination coupled with water remediation and molecular hydrogen production: a novel solar water-energy nexus†
Abstract
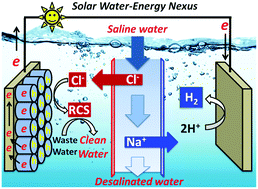
A novel sunlight-water-energy nexus technology is presented that combines the photoelectrocatalytic (PEC) desalination of saline water and desalination-driven wastewater remediation coupled with the production of molecular hydrogen (H2) from water. To accomplish this, morphologically tailored TiO2 nanorod (TNR) and hydrogen-treated TNR (H-TNR) array photoanodes are placed in an anode cell and Pt foils are located in a cathode cell, while a middle cell containing saline water (0.17 M NaCl) faces these cells through anion and cation exchange membranes, respectively. Upon irradiation by simulated sunlight (AM 1.5G, 100 mW cm−2), the photogeneration of charge carriers initiates the transport of chloride and sodium in the middle cell to the anode and cathode cells, respectively, leading to the desalination of saline water. The chloride in the anode cell is converted to reactive chlorine species (RCS), which effectively decompose urea to N2 as a primary product (>80%), while the sodium in the cathode cell accelerates the H2 production from water with a Faradaic efficiency of ∼80%. The PEC performance of the H-TNR photoanodes is superior to that of the TNR in the anodic and cathodic processes because of the reduced charge transfer resistance and sub-nanosecond charge transfer kinetics (∼0.19 ns), leading to a specific energy consumption of 4.4 kW h m−3 for 50% desalination, with an energy recovery of ∼0.8 kW h m−3. The hybrid system is found to operate for a period of ∼60 h with natural seawater, and virtually all the photoanodes are shown to be capable of driving the hybrid process. Although tested as a proof-of-concept, the present technology opens up a novel field involving a sunlight-water-energy nexus, promising high efficiency desalination and the desalination-driven remediation of water with simultaneous H2 production.


 Please wait while we load your content...
Please wait while we load your content...