Heavier pnictinidene gold(i) complexes†
Abstract
N,C,N-Chelated pnictinidenes ArE [where E = As, Sb or Bi; Ar = 2,6-(tBuN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)2C6H3] were used as ligands for the coordination of various gold(I) complexes. Thus, the reaction of ArE with [AuCl(Me2S)] gave complexes [AuCl(ArE)] [where E = As (1) or Sb (2)] that exhibited only limited stability in solution. By contrast, the reaction of ArBi with [AuCl(Me2S)] led to the immediate deposition of gold metal and the oxidation of the bismuth atom giving ArBiCl2. The treatment of a tetrameric gold alkynyl complex [Au(C
CH)2C6H3] were used as ligands for the coordination of various gold(I) complexes. Thus, the reaction of ArE with [AuCl(Me2S)] gave complexes [AuCl(ArE)] [where E = As (1) or Sb (2)] that exhibited only limited stability in solution. By contrast, the reaction of ArBi with [AuCl(Me2S)] led to the immediate deposition of gold metal and the oxidation of the bismuth atom giving ArBiCl2. The treatment of a tetrameric gold alkynyl complex [Au(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)]4 with ArAs and ArSb gave ionic compounds [Au(ArAs)2]+[Au2(C
CPh)]4 with ArAs and ArSb gave ionic compounds [Au(ArAs)2]+[Au2(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)3]− [denoted as 3+[Au2(C
CPh)3]− [denoted as 3+[Au2(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)3]−] and [Au(ArSb)2]+[Au(C
CPh)3]−] and [Au(ArSb)2]+[Au(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)2]− [denoted as 4+[Au(C
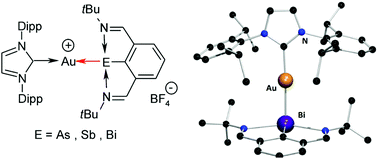
CPh)2]− [denoted as 4+[Au(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)2]−], respectively. Finally, the reaction of ArE with the carbene gold(I) complex [Au(IPr)(MeCN)]+[BF4]− [where IPr = 1,3-bis(2,6-diisopropylphenyl)imidazolin-2-ylidene, MeCN = acetonitrile] produced ionic complexes [Au(IPr)(ArE)]+[BF4]− [for cations: E = As (5+), Sb (6+) or Bi (7+)]. All complexes were characterized using 1H and 13C NMR, high mass accuracy electrospray ionization mass spectrometry (ESI-MS), IR and Raman spectroscopy and (except for 1) by single-crystal X-ray diffraction analysis. Furthermore, the structure and bonding of both neutral and ionic complexes with different coordination patterns have also been investigated in detail using a Density Functional Theory (DFT) computational approach.
CPh)2]−], respectively. Finally, the reaction of ArE with the carbene gold(I) complex [Au(IPr)(MeCN)]+[BF4]− [where IPr = 1,3-bis(2,6-diisopropylphenyl)imidazolin-2-ylidene, MeCN = acetonitrile] produced ionic complexes [Au(IPr)(ArE)]+[BF4]− [for cations: E = As (5+), Sb (6+) or Bi (7+)]. All complexes were characterized using 1H and 13C NMR, high mass accuracy electrospray ionization mass spectrometry (ESI-MS), IR and Raman spectroscopy and (except for 1) by single-crystal X-ray diffraction analysis. Furthermore, the structure and bonding of both neutral and ionic complexes with different coordination patterns have also been investigated in detail using a Density Functional Theory (DFT) computational approach.


 Please wait while we load your content...
Please wait while we load your content...