Tailoring the structure, pH sensitivity and catalytic performance in Suzuki–Miyaura cross-couplings of Ln/Pd MOFs based on the 1,1′-di(p-carboxybenzyl)-2,2′-diimidazole linker†
Abstract
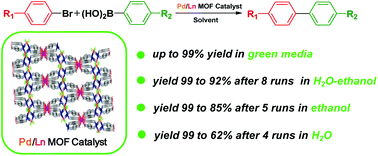
An array of heterobimetallic Pd/Ln MOFs (1–4) with Sm, Eu, Tb, Dy as preferred metal nodes and 1,1′-di(p-carboxybenzyl)-2,2′-diimidazole (H2L) as a fairly suitable bifunctional organic linker have been synthesized, fully characterized and tested as catalysts in cross-coupling reactions. These robust MOFs, ensuring a uniform distribution of Pd, showed excellent stability in air and high catalytic activity in Suzuki–Miyaura reactions conducted in neat water, neat ethanol as well as water–ethanol mixture. Depending on the solvent, complex 1 could be effectively recycled 4–8 times without significant loss of catalytic activity. Importantly, this complex was found to be pH responsive in a reversible way, enabling convenient recovery from acidic aqueous solutions, indicating good recyclability as well as environment-friendly separation of the metal residues after the reaction.


 Please wait while we load your content...
Please wait while we load your content...