Four-coordinated see-saw N-(aryl)-2-(propan-2-ylidene)hydrazinecarbothioamide complexes of nickel(ii), copper(ii) and zinc(ii) and their propensity for catalytic cyclisation†
Abstract
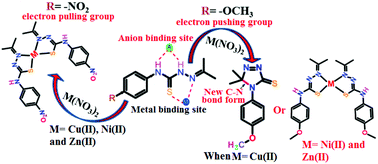
A series of mononuclear complexes of divalent nickel and zinc with N-(4-methoxyphenyl)-2-(propan-2-ylidene)hydrazine carbothioamide (H2Lmethoxy) as well as with N-(4-nitrophenyl)-2-(propan-2-ylidene)hydrazine carbothioamide (H2Lnitro) have been structurally characterised. Among these two ligands, H2Lnitro formed an analogous copper(II) complex to that of nickel and zinc, whereas H2Lmethoxy undergoes a catalytic cyclisation reaction in the presence of copper(II) nitrate trihydrate. Control experiments based on ESR have revealed that copper(II) is reduced to copper(I) and reverts back to copper(II) as the cyclisation reaction proceeds, generating a catalytic reaction. The crystal structures of H2Lnitro and H2Lmethoxy show that the plane of the phenyl ring with respect to the plane of the hydrazine-containing unit in H2Lnitro is more or less coplanar, whereas the H2Lmethoxy molecule is non-planar with a 67.05° angle between these planes. DFT calculations have shown a large difference in the localisation of electrons in the HOMO of the two ligands; the HOMO of H2Lmethoxy is spread over the aromatic ring, which facilitates involvement of the ring in the formation of a C–N bond through a single electron transfer to a copper(II) ion. This cyclic product has a distinguishable absorption maximum at 299 nm, which makes it possible to detect copper ions over other first row transition metal ions and alkali metal ions. On the other hand, the copper(II) complex of H2Lnitro shows a characteristic absorption at 395 nm in the presence of fluoride ions, whereas the free ligand with fluoride shows absorption at 418 nm, which shows that the interactions of the ligand with fluoride and with the corresponding copper complex widely differ.


 Please wait while we load your content...
Please wait while we load your content...