Triphenylstannyl((arylimino)methyl)benzoates with selective potency that induce G1 and G2/M cell cycle arrest and trigger apoptosis via ROS in human cervical cancer cells†
Abstract
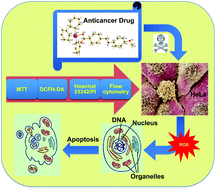
Metal complexes with organelle specificity and potent but selective cytotoxicity are highly desirable. A novel series of triphenylstannyl 4-((arylimino)methyl)benzoates (2–8) were obtained by the reactions of triphenylstannyl 4-formylbenzoate [Ph3Sn(L1)] 1 with primary aromatic amines. Two representative compounds (10, 11) were also synthesized by reacting aqua-triphenylstannyl 2-formylbenzoate [Ph3Sn(L9)(H2O)] (9) with aniline and p-fluoroaniline, respectively. These compounds were characterized by elemental analysis, IR and 1H, 13C and 119Sn NMR spectroscopy, as well as single-crystal X-ray diffraction for compounds 5, 7–11 and three pro-ligands. The in vitro cytotoxic activities of 1–11 were assessed using the MTT tetrazolium dye assay against HeLa (human cervical) and MDA-MB-231 (breast) cancer cells, with IC50 values revealing high activity. Compared to cisplatin, compounds 1–11 exhibited enhanced cytotoxic efficacy, indicating their potential as potent anticancer agents. Among these, 1 and 5 demonstrated maximum inhibition in HeLa cells, with negligible effect on normal human embryonic kidney (HEK) cells. The combined results of the DCFH-DA dye and Hoechst 33342/PI nuclear staining assays, along with flow cytometry analysis, show that they possess a dual mode of action: They induced apoptotic cell death, attributable to the tin-assisted generation of reactive oxygen species. Cell cycle analyses indicated that compounds 1 and 5 exhibit cell growth inhibition and may cause turbulences in the G1 and G2/M phases.


 Please wait while we load your content...
Please wait while we load your content...