Oxygenate formation over K/β-Mo2C catalysts in the Fischer–Tropsch synthesis†
Abstract
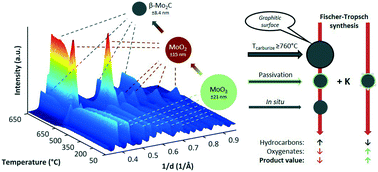
The Fischer–Tropsch (FT) process, producing long chained waxes and transportation fuels, is competing with fuels derived from crude oils and its profitability is therefore dependent on the global oil price. However, increasing the value of synthesized products could render the profitability of the FTS independent of the common fluctuations in the commodity price (which are mostly due to global political trends and only to a lesser extent due to market requirements). One way to achieve this, is to target the more valuable products of the Fischer–Tropsch spectrum, for example oxygenates. This study investigates the effect of synthesis protocols on the surface characteristics of molybdenum carbide and the use of potassium promoted Mo2C as a catalyst for higher oxygenate (C2+ oxygenates) synthesis in CO hydrogenation. A graphitic surface layer was observed with TEM, XPS and Raman analysis for Mo2C samples carburized at ≥760 °C. The graphitic carbon, blocking active sites and therefore significantly lowering catalytic activity, could be partially removed by means of a temperature programmed hydrogenation, forming methane. An unpromoted β-Mo2C catalyst, carburized at 630 °C, reached CO conversions up to ±40% at the conditions applied. Initial 6.2 wt% K/Mo promotion of the catalyst with potassium showed a significant drop in catalyst activity, however, an increase in potassium content did not further decrease catalyst activity. The selectivity towards oxygenates was enhanced, yet it has a certain optimum with regards to promotor concentration. Simultaneously, the oxygenate distribution shifted towards higher alcohols. The initial methanol content in the total oxygenate product was around 60 C% and decreased to approximately 20 C% upon potassium promotion.


 Please wait while we load your content...
Please wait while we load your content...