Tuning the micromorphology and exposed facets of MnOx promotes methyl ethyl ketone low-temperature abatement: boosting oxygen activation and electron transmission†
Abstract
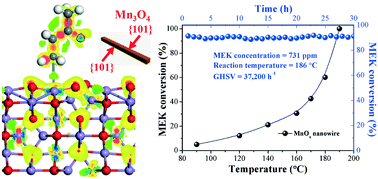
MnOx oxides with different morphologies (nanowire (MnOx-W), nanocube (MnOx-C), nanorod (MnOx-R), and nanosphere (MnOx-S)) and exposed facets were synthesized via a solvothermal method. The catalytic performance of the synthesized MnOx materials for methyl ethyl ketone (MEK) destruction was investigated. Results show that the activity of MnOx-W with highly exposed {101} facets of Mn3O4 is superior to that of MnOx-C, MnOx-R, and MnOx-S exposing {321} facets of Mn2O3, {110} facets of MnO2, and {101} and {112} facets of Mn3O4, respectively. MEK can be completely mineralized into CO2 at 195 °C over MnOx-W under a relatively high gas hourly space velocity of 37 200 h−1, which is even better than some typical noble metal loaded catalysts. The lowest apparent activation energy of MnOx-W (27.7 kJ mol−1) for MEK destruction also confirms its excellent catalytic activity. Density functional theory (DFT) results reveal that the {101} facets of Mn3O4 have the highest MEK adsorption energy (0.79 eV), which indicates that MEK molecules have a high affinity to adsorb onto the MnOx-W surface, promoting the oxidation process of MEK. In situ DRIFTS and TPSR results indicate that the mineralization of MEK into CO2 over MnOx-W goes through an oxidation route with diacetyl as the primary intermediate. We found that the highly exposed {101} active facets, abundant oxygen vacancies, and excellent low-temperature reducibility are responsible for the superior oxidation performance of MnOx-W. This finding may bring new insights into the designing of highly effective catalysts and has implications for a wide range of reactions not limited to MEK oxidation.


 Please wait while we load your content...
Please wait while we load your content...