Hierarchical TiO2 nanowire/microflower photoanode modified with Au nanoparticles for efficient photoelectrochemical water splitting†
Abstract
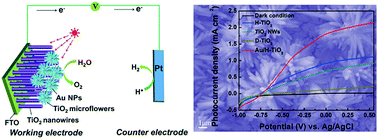
An efficient photoelectrochemical water splitting system is developed based on a hierarchical TiO2 nanowire/microflower (H-TiO2) photoanode, which is synthesized via a one-step hydrothermal process. The H-TiO2 structure is formed via three primary self-assembly processes and simultaneously offers excellent ultraviolet light absorption capacity and large specific surface area. The H-TiO2 photoanode achieves a 1.3 fold enhancement in photocurrent density compared to its TiO2 nanowire-based counterpart at 1.23 V vs. reversible hydrogen electrode (the theoretical potential for water electrolysis). More strikingly, by incorporating the surface plasmon resonance (SPR) effect from Au nanoparticles (NPs), the resulting Au/H-TiO2-based photoanodes (Au/H-TiO2) exhibit an about two-fold enhancement in photocurrent density under both simulated sunlight and visible-light illumination. Moreover, the maximum photoconversion efficiency of Au/H-TiO2 gives three fold enhancement compared to that in the absence of Au NPs. Significantly, Au NPs incorporated on the H-TiO2 surface also serve as co-catalysts like Pt under white-light illumination, reducing the onset potential by about 0.15 V and the current saturation potential by about 0.18 V. The enhanced photoactivity under white light is due to the co-action of electron transfer from the efficient carrier separation of H-TiO2 (ultraviolet part) and Au to H-TiO2 under the SPR effect (visible part). The present study provides a new strategy for designing and fabricating TiO2-based devices with favorable energy conversion efficiency for photoelectrochemical water splitting.


 Please wait while we load your content...
Please wait while we load your content...