Base-promoted hydrolytic dehydrogenation of ammonia borane catalyzed by noble-metal-free nanoparticles†
Abstract
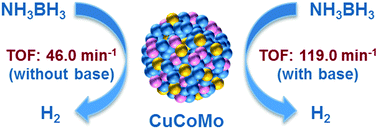
Hydrogen is a potentially ideal alternative energy source for both environmental and economic reasons. The development of sustainable, efficient, and economical catalysts towards hydrogen generation from hydrogen storage materials (e.g., ammonia borane, AB) has been one of the active and challenging research areas. In this work, noble-metal-free CuCoMo nanoparticles (NPs) without any surfactant or support have been prepared via a facile co-reduction method at room temperature and used as highly efficient catalysts for hydrogen generation from an aqueous AB solution. Compared to their monometallic and bimetallic counterparts, the obtained trimetallic Cu0.72Co0.18Mo0.1 NPs exhibit the highest catalytic activity for the hydrolysis of AB with a total turnover frequency (TOF) value of 46.0 min−1 at room temperature under ambient atmosphere. The Cu0.72Co0.18Mo0.1 NPs only needed 0.63 min to completely hydrolyse AB in the presence of a basic solution (NaOH, 1 M) with a TOF value as high as 119.0 min−1 at room temperature, which is among the highest values of all the noble-metal-free catalysts ever reported for the same reaction, wherein NaOH plays an important role in improving the catalytic activity. In addition, it is found that the hydrogen generation rate can be improved by adding a base (e.g., NaOH, KOH, etc.) into the reaction solution, while the presence of NH4+ species (e.g., NH3·H2O, NH4Cl, etc.) is unfavorable for the hydrolysis of AB.


 Please wait while we load your content...
Please wait while we load your content...