Steel-based electrocatalysts for efficient and durable oxygen evolution in acidic media†
Abstract
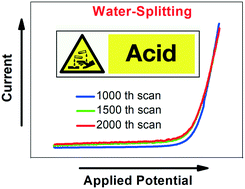
High overpotentials, particularly an issue of common anode materials, hamper the process of water electrolysis for clean energy generation. Thanks to immense research efforts, up-to-date oxygen evolution electrocatalysts based on earth-abundant elements work efficiently and stably in neutral and alkaline regimes. However, non-noble metal-based anode materials that can withstand low pH regimes are considered to be an indispensable prerequisite for the water splitting to succeed in the future. All oxygen-evolving electrodes working durably and actively in acids contain Ir at least as an additive. Due to their scarcity and high acquisition costs, noble elements like Pt, Ru and Ir need to be replaced by earth-abundant elements. We have evaluated a Ni-containing stainless steel for use as an oxygen-forming electrode in diluted H2SO4. Unmodified Ni42 steel showed a significant weight loss after long-term OER polarization experiments. Moreover, a substantial loss of the OER performance of the untreated steel specimen seen in linear sweep voltammetry measurements turned out to be a serious issue. However, upon anodization in LiOH, Ni42 alloy was rendered in OER electrocatalysts that exhibit, under optimized synthesis conditions, stable overpotentials down to 445 mV for a current density of 10 mA cm−2 at pH 0. Even more importantly, the resulting material has been proven to be robust upon long-term usage (weight loss: 20 μg mm−2 after 50 ks of chronopotentiometry at pH 1) towards OER in H2SO4. Our results suggest that electrochemical oxidation of Ni42 steel in LiOH (sample Ni42Li205) results in the formation of a metal oxide-containing outer zone that supports solution route-based oxygen evolution in the acidic regime accompanied by good stability of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...