Reaction mechanism for the conversion of methanol to olefins over H-ITQ-13 zeolite: a density functional theory study†
Abstract
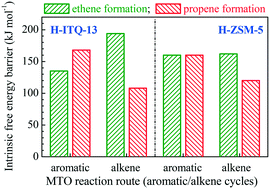
It has been found in recent years that H-ITQ-13 zeolite as a catalyst performs excellently in the conversion of methanol to olefins (MTO); however, the relationship between the catalytic performance and its unique pore structure remains rather ambiguous. In this work, the reaction mechanism of MTO over H-ITQ-13 was investigated by density functional theory considering dispersive interactions (DFT-D). The results illustrate that both the aromatic and alkene cycles are viable for MTO over H-ITQ-13. Via the aromatic cycle, the selectivity to ethene is higher than that to propene (with pentamethylbenzene (5MB) and 1,4-dimethylnaphthalene (2MN) as the active hydrocarbon pool (HCP) species), whereas via the alkene cycle (with lower alkenes as the HCP species), propene is the primary product. The alkene cycle takes priority over the aromatic cycle, since the transition states formed in the alkene cycle can be well stabilized in the three-dimensional framework of small pores in H-ITQ-13; as a result, high selectivity to propene can be achieved for MTO over H-ITQ-13. The insights shown in this work help to clarify the reaction mechanism of MTO over H-ITQ-13 and reveal the relationship between the catalytic performance and the unique pore structure of H-ITQ-13, which is of great benefit to the development of better MTO catalysts and reaction processes with high selectivity to propene.


 Please wait while we load your content...
Please wait while we load your content...