Dynamical crossover of water confined within the amphiphilic nanocores of aggregated amyloid β peptides
Abstract
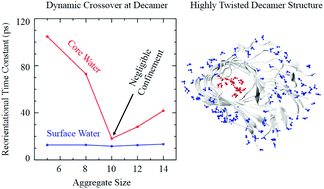
It is believed that the self-assembly of amyloid beta (Aβ) peptides in the brain is the cause of Alzheimer's disease. Atomistic molecular dynamics simulations of aqueous solutions of Aβ protofilaments of different sizes at room temperature have been carried out to explore the dynamic properties of water confined within the core and at the exterior surface of the protofilaments. Attempts have been made to understand how the non-uniform distortion of the protofilaments associated with their structural crossover influences the diffusivity and the hydrogen bonding environment of the confined water molecules. In contrast to the homogeneous solvent dynamical environment at the exterior surface, the calculations revealed heterogeneously restricted motions of water confined within the distorted cores of the protofilaments. Importantly, it is demonstrated that the structural crossover of the aggregates observed for the decamer is associated with a dynamical transition of water confined within its core. A direct one-to-one correlation between the heterogeneously restricted core water motions and the kinetics of the breaking and formation of hydrogen bonds quantitatively demonstrated that a modified hydrogen bond arrangement within the cores of higher order Aβ protofilaments is the origin behind the crossover in core water mobility. A fraction of the water molecules forming short-lived water–water hydrogen bonds within the core of the crossover protofilament decamer are believed to diffuse away easily from the core and thus play a crucial role in further growth of the protofilament by facilitating the binding of new peptide monomers.


 Please wait while we load your content...
Please wait while we load your content...