Towards the low-sensitive and high-energetic co-crystal explosive CL-20/TNT: from intermolecular interactions to structures and properties
Abstract
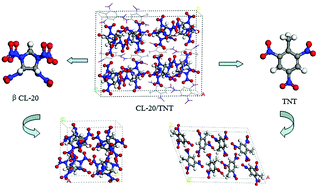
Employing molecular dynamic (MD) simulations and solid-state density functional theory (DFT), we carried out thorough studies to understand the interaction-structure–property interrelationship of the co-crystal explosive 1 : 1 CL-20 : TNT. Our results revealed that the co-crystallization of CL-20 and TNT molecules enhances the intermolecular binding forces, where the main driving force for the formation of the co-crystal CL-20/TNT comes from H⋯O and C⋯O interactions, while O⋯O contributes to the co-crystal stabilization. Furthermore, we also used the concept of atoms in molecule (AIM) and the reduced density gradient (RDG) to describe the spatial arrangements and interactions of co-crystal compositions, which showed that although the adjoining TNT molecules possess two symmetry groups and the adjoining CL-20 molecules possess the same symmetry group, their interactions are not identical. These spatial arrangements provide a good reference to the formation of other co-crystals. When the obtained structural and detonation properties of the three crystals were compared, it was observed that the CL-20/TNT co-crystal achieved the desirable properties of explosives, i.e., low-sensitivity and high-energy, possessing the advantages of both CL-20 and TNT explosives.


 Please wait while we load your content...
Please wait while we load your content...