Unravelling the influence of carbon dioxide on the adsorptive recovery of butanol from fermentation broth using ITQ-29 and ZIF-8†
Abstract
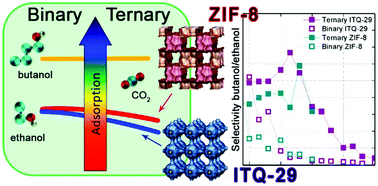
The vapor phase adsorption of butanol from ABE fermentation at the head space of the fermenter is an interesting route for the efficient recovery of biobutanol. The presence of gases such as carbon dioxide that are produced during the fermentation process causes a stripping of valuable compounds from the aqueous into the vapor phase. This work studies the effect of the presence of carbon dioxide on the adsorption of butanol at a molecular level. With this aim in mind Monte Carlo simulations were employed to study the adsorption of mixtures containing carbon dioxide, butanol and ethanol. Molecular models for butanol and ethanol that reproduce experimental properties of the molecules such as polarity, vapor–liquid coexistence or liquid density have been developed. Pure component isotherms and heats of adsorption have been computed and compared to experimental data to check the accuracy of the interacting parameters. Adsorption of butanol/ethanol mixtures has been studied in absence and presence of CO2 on two representative materials, a pure silica LTA zeolite and a hydrophobic metal–organic framework ZIF-8. To get a better understanding of the molecular mechanism that governs the adsorption of the targeted mixture in the selected materials, the distribution of the molecules inside the structures was analyzed. The combination of these features allows obtaining a deeper understanding of the process and to identify the role of carbon dioxide in the butanol purification process.


 Please wait while we load your content...
Please wait while we load your content...