Negative effective Li transference numbers in Li salt/ionic liquid mixtures: does Li drift in the “Wrong” direction?†
Abstract
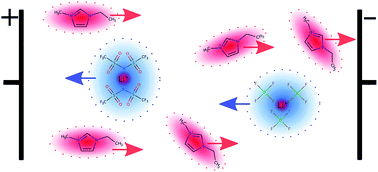
The electrophoretic mobilities μ of all ion species in the lithium salt/ionic liquid mixtures LiTFSA/EmimTFSA and LiBF4/EmimBF4 are determined by 1H, 19F and 7Li electrophoretic NMR. The average drift direction of Li is identical to that of the anions TFSA− or BF4−. This proves a correlated ion motion of Li with the anions in negatively charged Li-containing clusters in both systems. The effective charge of these clusters is determined as −1, or −2 in the system with TFSA or BF4, respectively, pointing at the existence of [Li(TFSA)2]− or [Li(BF4)3]2−. This behavior is described by a negative effective transference number of Li, resulting in a negative contribution of Li ions to the overall conductivity. Li effective transference numbers are in the range of −0.04 to −0.02, depending on Li salt concentration and anion type. Transference numbers thus clearly deviate from apparent transference numbers estimated from diffusion coefficients, as an effect of a vehicular transport mechanism. This has important implications for the mechanism of Li mass transport in Li ion batteries as the drift of charged clusters has to be overcompensated by diffusive mass transport of neutral, Li-containing aggregates.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...