Electrochemical nanoarchitectonics through polyaminobenzylamine–dodecyl phosphate complexes: redox activity and mesoscopic organization in self-assembled nanofilms†
Abstract
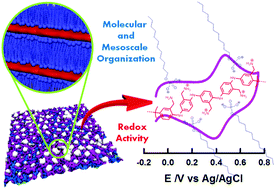
Molecular design and preparation of redox active films displaying mesoscopic levels of organization represents one of the most actively pursued research areas in nanochemistry. These mesostructured materials are not only of great interest at the fundamental level because of their unique properties but they can also be employed for a wide range of applications such as electrocatalysts, electronic devices, and electrochemical energy conversion and storage. Herein, we introduce a simple and straightforward strategy to chemically modify electrode surfaces with self-assembled electroactive polyelectrolyte–surfactant complexes. These assemblies are composed of amino-appended polyaniline and monododecyl phosphate. The complexes were deposited by spin-coating and the films were characterized by spectroscopic and X-ray-based techniques: XRR, GISAXS, WAXS, and XPS. The films presented a well-defined lamellar structure, directed by the strong interaction between the phosphate groups and the positively charged amine groups in the polyelectrolyte. These films also displayed intrinsic electroactivity in both acidic and neutral solutions, showing that the polymer remains electroactive and ionic transport is still possible through the stratified and hydrophobic coatings. The stability and enhanced electroactivity in neutral solutions make these assembled films promising building blocks for the construction of nanostructured electrochemical platforms.


 Please wait while we load your content...
Please wait while we load your content...