Defect chemistry and electrical properties of garnet-type Li7La3Zr2O12†
Abstract
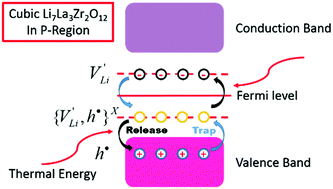
Garnet-type cubic Li7La3Zr2O12 exhibits one of the highest lithium-ion conductivity values amongst oxides (up to ∼2 mS cm−1 at room temperature). This compound has also emerged as a promising candidate for solid electrolytes in all-solid-state lithium batteries, due to its high ionic conductivity, good chemical stability against lithium metal, and wide electrochemical stability window. Defect chemistry of this class of materials, although less studied, is critical to the understanding of the nature of ionic conductivity and predicting the properties of grain boundaries and heterogeneous solid interfaces. In this study, the electrical properties of nominally undoped cubic Li7La3Zr2O12 are characterized as a function of temperature and pO2 using a suite of AC impedance and DC polarization techniques. The formation of ionic defects and defect pairs as well as their impact on the transport properties are discussed, and a Brouwer-type diagram is constructed.


 Please wait while we load your content...
Please wait while we load your content...