Heat of adsorption, adsorption stress, and optimal storage of methane in slit and cylindrical carbon pores predicted by classical density functional theory
Abstract
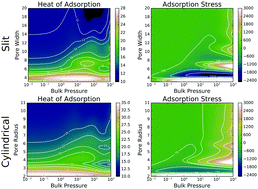
Temperature, pressure and pore-size dependences of the heat of adsorption, adsorption stress, and adsorption capacity of methane in simple models of slit and cylindrical carbon pores are studied using classical density functional theory (CDFT) and grand-canonical Monte-Carlo (MC) simulation. Studied properties depend nontrivially on the bulk pressure and the size of the pores. Heat of adsorption increases with loading, but only for sufficiently narrow pores. While the increase is advantageous for gas storage applications, it is less significant for cylindrical pores than for slits. Adsorption stress and the average adsorbed fluid density show oscillatory dependence on the pore size and increase with bulk pressure. Slit pores exhibit larger amplitude of oscillations of the normal adsorption stress with pore size increase than cylindrical pores. However, the increase of the magnitude of the adsorption stress with bulk pressure increase is more significant for cylindrical than for slit pores. Adsorption stress appears to be negative for a wide range of pore sizes and external conditions. The pore size dependence of the average delivered density of the gas is analyzed and the optimal pore sizes for storage applications are estimated. The optimal width of slit pore appears to be almost independent of storage pressure at room temperature and pressures above 10 bar. Similarly to the case of slit pores, the optimal radius of cylindrical pores does not exhibit much dependence on the storage pressure above 15 bar. Both optimal width and optimal radii of slit and cylindrical pores increase as the temperature decreases. A comparison of the results of CDFT theory and MC simulations reveals subtle but important differences in the underlying fluid models employed by the approaches. The differences in the high-pressure behaviour between the hard-sphere 2-Yukawa and Lennard-Jones models of methane, employed by the CDFT and MC approaches, respectively, result in an overestimation of the heat of adsorption by the CDFT theory at higher loadings. However, both adsorption stress and adsorption capacity appear to be much less sensitive to the differences between the models and demonstrate excellent agreement between the theory and the computer experiment.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...