11-Azaartemisinin cocrystals with preserved lactam : acid heterosynthons†
Abstract
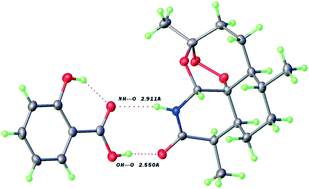
11-Azaartemisinin (11-Aza) is a potent anti-malarial drug more readily able to form cocrystals than its parent compound. 13 new 1 : 1 and 2 : 1 cocrystal phases from 25 mono- and bi-functional acids are reported here in excellent yield and purity by liquid assisted grinding. X-ray structures of several of these cocrystals reveal R22(8) heterosynthons with short OH–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C H-bonds ≤2.60 Å between the 11-Aza lactam and the coformer acid groups. The new phases can show enhanced aqueous solubility of 11-Aza of 3× after 12 h. Notably diastereospecific cocrystal formation can occur, with the DL-mandelic acid system yielding solely the 11-Azaart : D-mandelic acid 1 : 1 product.
C H-bonds ≤2.60 Å between the 11-Aza lactam and the coformer acid groups. The new phases can show enhanced aqueous solubility of 11-Aza of 3× after 12 h. Notably diastereospecific cocrystal formation can occur, with the DL-mandelic acid system yielding solely the 11-Azaart : D-mandelic acid 1 : 1 product.


 Please wait while we load your content...
Please wait while we load your content...