Polymorphs of oxindole as the core structures in bioactive compounds†
Abstract
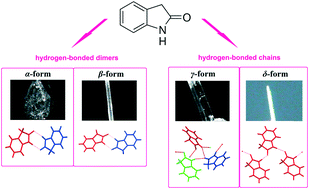
A new polymorph of oxindole (termed as “δ-form”) has been found and characterized by single-crystal X-ray diffraction. Its structural properties were compared with those of the previously reported polymorphs (the α-, β- and γ-forms). This polymorphic form was crystallized for the first time but could not be reproduced. Thus, the δ-form can serve as an example of a “disappearing polymorph”. As a result, the three previously reported α-, β- and γ-forms have now been characterized by powder X-ray diffraction, vibrational spectroscopy (infrared and Raman) and calorimetry (differential scanning calorimetry). In the structures of the earlier known α- and β-forms, N–H⋯O hydrogen bonds connect the molecules into dimers, while the crystals of the γ- and δ-forms contain hydrogen-bonded chains of associated molecules. An analysis of the Hirshfeld surfaces and fingerprint plots has also been performed to study the intermolecular interactions in the crystal network of each polymorph. The spectroscopic and thermal investigations showed that the polymorphs convert into the α-form, which indicates that the α-form is the most stable at ambient temperature. Additionally, the α- and β-forms are enantiotropically-related phases, whereas the γ-form is monotropically related to the α-form. Unexpectedly, the β- and γ-forms were found to be unstable under Cu Kα radiation and both gradually transformed into the α-form with the γ-phase being more stable.


 Please wait while we load your content...
Please wait while we load your content...