Expanding the chemistry of ring-fused 1,4-diphosphinines by stable mono anion formation†
Abstract
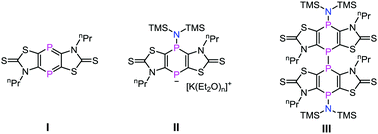
A new sulfur-enriched tricyclic 1,4-diphosphinine (2) was synthesized and novel reactivity studies on the phosphorus heterocycle were performed: a weak anionic nucleophile (KHMDS) adds selectively thus forming a stable anionic 1,4-diphosphinine derivative (3b) which was fully characterized. The substitution potential of 3b was demonstrated using Ph2PCl to give 4b, while oxidation of 3b using elemental iodine furnished cleanly the P–P coupling product 5.


 Please wait while we load your content...
Please wait while we load your content...