Ionic liquid syntheses via click chemistry: expeditious routes toward versatile functional materials
Abstract
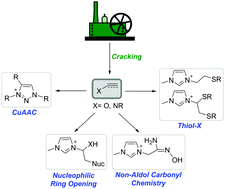
Since the introduction of click chemistry by K. B. Sharpless in 2001, its exploration and exploitation has occurred in countless fields of materials sciences in both academic and industrial spheres. Click chemistry is defined as an efficient, robust, and orthogonal synthetic platform for the facile formation of new carbon–heteroatom bonds, using readily available starting materials. Premier examples of click reactions are copper(I)-catalyzed azide–alkyne Huisgen cycloaddition (CuAAC) and the thiol–X (X = ene and yne) coupling reactions to form C–N and C–S bonds, respectively. The emphasis of this review is centered on the rapidly expanding area of click chemistry-mediated synthesis of functional ionic liquids via CuAAC, thiol–X and oxime formation, and selected examples of nucleophilic ring-opening reactions, while offering some thoughts on emerging challenges, opportunities and ultimately the evolution of this field. Click chemistry offers tremendous opportunities, and introduces intriguing perspectives for efficient and robust generation of tailored task-specific ionic liquids – an important class of soft materials.
- This article is part of the themed collection: Ionic Liquids in the Synthesis, Fabrication, and Utilization of Materials and Devices


 Please wait while we load your content...
Please wait while we load your content...