Probing the speciation of quaternary ammonium polybromides by voltammetric tribromide titration†
Abstract
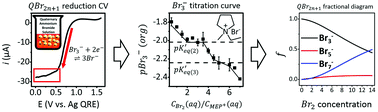
The speciation of quaternary ammonium polybromides (QBr2n+1) was quantitatively determined by voltammetric tribromide titration on a Pt ultramicroelectrode (UME). The concentration of Br3− in a QBr2n+1-water mixed solution (QBr2n+1-WMS) was electrochemically estimated by measuring the steady state current associated with the electro-reduction of Br3− in a linear sweep voltammogram (LSV). The pBr3− titration curves of QBr2n+1-WMSs show 2–4 plateaus, each of which relates to the formation of QBr2n+1 from Br3− and Br2. The values of pBr3− at these plateaus can be regarded as corrected equilibrium constants of QBr2n+1, K′eq(n), which is Keq(n)/γ±, where γ± is a mean activity coefficient in QBr2n+1-WMS. Based on the estimated K′eq(n), fractional diagrams of QBr2n+1 were obtained, which gave information on QBr2n+1 speciation.


 Please wait while we load your content...
Please wait while we load your content...