Voltammetric determination of N-hydroxysuccinimide at conductive diamond electrodes
Abstract
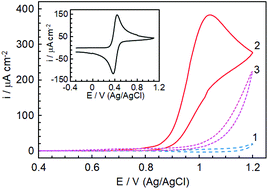
Boron-doped diamond (BDD) electrodes were used to investigate the possibility of detecting N-hydroxysuccinimide (NHS) by linear sweep anodic voltammetry. By increasing the pH the peak potential corresponding to NHS oxidation gradually decreases, suggesting that the electroactive species are the deprotonated ones. An exponential enhancement of the peak current with increasing pH was also observed, indicating that the overall process involves OH−-mediated regeneration of these species. The sweep rate effect, together with digital simulation, allowed ascribing the anodic peak to a mechanism consisting of a slow uptake of an electron from the deprotonated NHS species, followed by their catalytic regeneration through a fast chemical reaction. It was also found that in alkaline media the voltammetric response is suitable for analytical applications. A method was proposed for the determination of NHS in the concentration range 5 μM to 10 mM. The good analytical performance characteristics and the wide dynamic range of analytically useful response are also meaningful as they might come in useful in providing a basis for new, electrochemical, approaches for NHS quantification.


 Please wait while we load your content...
Please wait while we load your content...